An Institutional Approval certificate must be issued before you start your research project and is required for all studies.
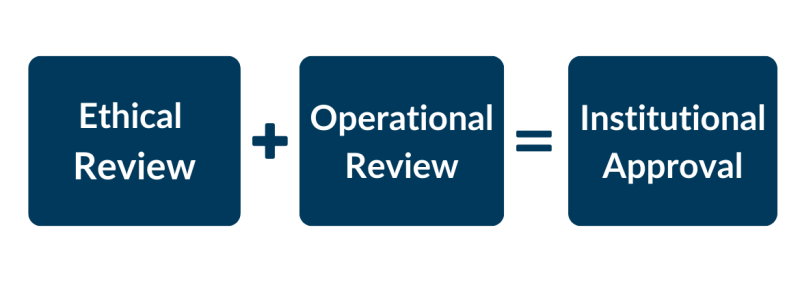
Ethical Review and Operational Review are separate processes at Island Health that will be conducted concurrently.
Operational Review is required for all studies. The review is conducted by the department directors, delegates and/or managers where you will be:
- accessing Island Health property, resources and/or facilities (including research conducted in clinics, use of radiation, pharmacy, laboratory and pathology services, and use of health records)
- involving and/or recruiting patients and staff and/or,
- utilizing resources, data, programs and/or services of Island Health, or its patients, clients, residents or staff
- and for team member affiliation, whereby you attest to not requesting or requiring any other Island Health data, access or services.
APPLY FOR OPERATIONAL REVIEW
The Island Health Research Services Portal contains all forms required for your research project at Island Health. Please fill out the forms completely, even if you have provided similar information in the ethics portion of the application. We must receive an operational application to be paired with your ethics application, whether you apply on the Island Health Research Services Portal or the PREP application on RISe.
Once your application is submitted, the Director(s) and/or Manager(s) of each department will receive an email advising them that there is a research application that requires their review. They can indicate in the review process whether they support the research study being conducted in their department.
The Operational Application form found in the Island Health Research Services Portal provides specific requests for support for research from:
- Contracts and Agreements
- Decision Support and Databases (access to Island Health data for research)
- Health Information Management (paper-based or electronic records)
- Heart Health
- Laboratory Medicine
- Medical Imaging
- Medical Device Reprocessing
- Pharmacy (including data stewardship)
- Privacy and Compliance
Recruitment Support for External Researchers
For Researchers who are external to Island Health or Research Ethics BC (your study is not harmonized in RISe/PREP)
If you are a researcher whose study has been approved by an external REB, you are requesting recruitment support only, and you are not requesting any services from Island Health, please follow these steps:
- Email ResearchEthics@islandhealth.ca
- Include "External researcher seeking recruitment support" in the subject line.
- Attach REB Certificate of Approval and copy of material(s) to be distributed.
The Research Ethics & Compliance Office will review and respond to your request.
FAQs
When can I start my research?
At Island Health, research can only begin when your Institutional Approval (IA) certificate is issued. Institutional Approval means both Ethical review and Operational review have been completed and approvals granted by all applicable boards and departments. When you have received your IA certificate, you can approach a department for a service; present your IA certificate and the accompanying email listing the approval departments.
When is Operational Review required?
Operational review is required for all studies. If your research impacts Island Health sites, employees, patients, data or other resources, or you have a team member using their Island Health affiliation, then you must submit an operational application in addition to your ethics application. Operational review runs concurrently with the ethical review.
How do I apply for Operational Review?
The application can be found on the Island Health Research Services Portal. Apply for an operational review.
